View John Dalton Diagram Pics. Though some of his conclusions were incorrect, his contributions were vital. John dalton uses the law of multiple proportions, the law of definite composition and the works of others to develop his postulates on the make up of matter.
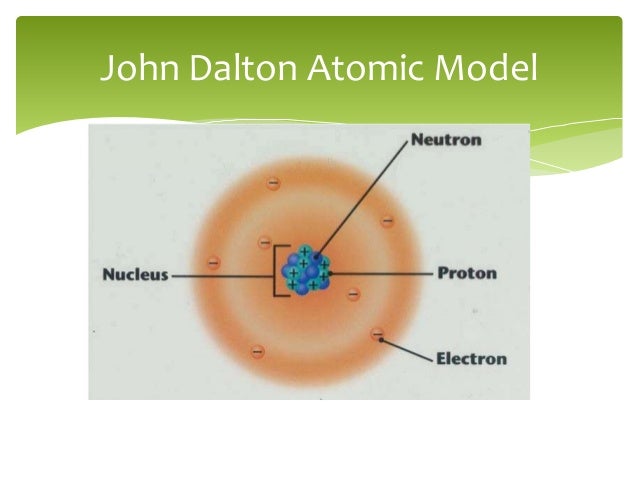
Dalton's atomic theory was a theory on the nature of matter which stated that all matter was made up of small, indivisible particles known as 'atoms'.
Atoms are small indivisible particles that combine to make compounds. Matter consists of indivisible particles called atoms. Here dalton shows how water molecules might arrange themselves when they are frozen in ice. Here dalton shows how water molecules might arrange themselves when they are frozen in ice.


